An oxygen vacancy-rich two-dimensional Au/TiO2 hybrid for synergistically enhanced electrochemical N2 activation and reduction†
Abstract
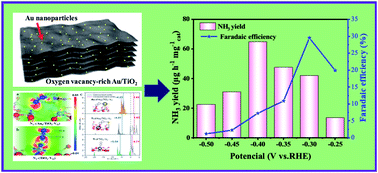
The electrochemical nitrogen reduction reaction (NRR) is emerging as a promising sustainable technique that can convert nitrogen (N2) to ammonia (NH3) under ambient conditions. However, the selectivity and electrocatalytic activity of the NRR obtained experimentally to date are still unsatisfactory. As a consequence, it remains a technical challenge to develop scalable, low-cost, and efficient NRR electrocatalysts. Herein, we present an oxygen vacancy (VO)-rich two-dimensional gold (Au)/titanium dioxide (TiO2) hybrid that acts as an advanced NRR electrocatalyst under ambient conditions. Electrocatalytic testing demonstrated that the Au/TiO2 hybrid catalyst delivered a promising NH3 yield of 64.6 μg h−1 mg−1 cat and a high faradaic efficiency of 29.5% at −0.40 V versus the reversible hydrogen electrode in acidic media. Experimental findings and theoretical calculations demonstrate that the strong electron-donating effect of VO in TiO2 was essential for N2 activation. Meanwhile, the introduction of Au promotes both the electrochemical and thermodynamic rate-limiting steps by adjusting the electronic structure of the active sites. Together, these effects greatly facilitate the activation and reduction of N2 under ambient conditions. The specific catalyst design strategy in our work represents an alternative avenue to produce other two-dimensional electrocatalysts with high NRR activity and selectivity through rational structural engineering.


 Please wait while we load your content...
Please wait while we load your content...