Li+ conducting garnet-type oxide sintering triggered by an H+/Li+ ion-exchange reaction†
Abstract
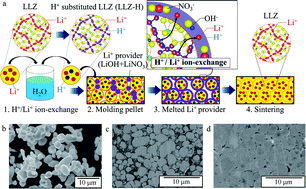
Solid-state batteries (SSBs) using a garnet-based oxide electrolyte, Li7La3Zr2O12 (LLZ), are attracting considerable attention as a future power storage solution with a promising higher safety level than that of the conventional lithium-ion batteries (LIBs) using liquid organic electrolytes due to the non-flammability of LLZ. However, the major obstacle in realizing oxide-based SSBs is their high-temperature fabrication process (e.g., above 600 °C), resulting in the formation of insulating impurities by the reaction between the cathode and electrolyte materials. Herein, we have demonstrated the fabrication of a solid-state Li/LLZ/Li(Ni1/3Co1/3Mn1/3)O2 battery at a remarkably low temperature (400 °C) using our invented “low temperature sintering triggered by an ion-exchange reaction (LTI)”. The LTI promotes element diffusion via limited ion exchange within a range that maintains the crystal structure, enabling a lower sintering temperature and shorter sintering time. The high capacity of the fabricated SSB was confirmed to be 127 mA h g−1 for the electrode and the operation temperature was increased up to 100 °C, where conventional LIBs with liquid electrolytes do not work due to the vaporization of the solvent.


 Please wait while we load your content...
Please wait while we load your content...