A composite solid polymer electrolyte incorporating MnO2 nanosheets with reinforced mechanical properties and electrochemical stability for lithium metal batteries†
Abstract
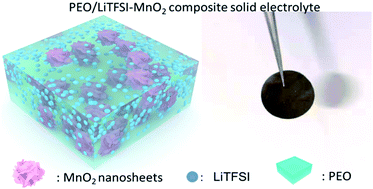
A solid polymer electrolyte is expected to be useful for safe and high energy density lithium-metal batteries owing to its good flexibility and high degree of safety. The development of a polyethylene oxide (PEO) based solid electrolyte is still restrained by low ionic conductivity and unsatisfactory mechanical strength. Since MnO2 could combine with PEO chains and Li ions could undergo long-range migration on MnO2 nanosheets, MnO2 nanoflakes are chosen as fillers to improve the electrochemical and mechanical properties of a solid polymer electrolyte. A PEO/MnO2 composite solid polymer electrolyte (CSPE) displays a higher lithium ion transference number (0.378), higher ionic conductivity (1.5 times higher at 60 °C) and better tensile strength (2.3 times) than a PEO solid electrolyte. Density functional theory calculations reflect the fact that the binding energy between the PEO/Li complex and MnO2 is small and there is easy desorption of Li from PEO and migration on MnO2 nanosheets, indicating enhanced lithium ion transport in the electrolyte system. A solid-state lithium metal battery using a PEO/MnO2 CSPE delivers higher capacity (143.5 mA h g−1 after 300 cycles) than an electrolyte without fillers (61.2 mA h g−1 after 90 cycles). Soft-package lithium metal batteries with an MnO2 CSPE reveal high safety after cutting, nail and bending tests.


 Please wait while we load your content...
Please wait while we load your content...