Free-standing 3D nitrogen–carbon anchored Cu nanorod arrays: in situ derivation from a metal–organic framework and strategy to stabilize lithium metal anodes†
Abstract
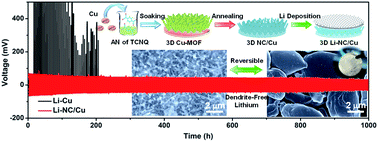
Lithium (Li)-metal is considered to be the most promising candidate as an anode to design higher energy density lithium batteries because of its extremely high specific capacity (3860 mA h g−1) and lowest redox potential (−3.04 V vs. SHE). However, the safety concerns of lithium dendrites, low coulombic efficiency, and infinite volume variation remain challenging for practical applications. Herein, a free-standing 3D nitrogen–carbon anchored Cu nanorod array (NC/Cu) as a new current collector is introduced. It was in situ derived from a metal–organic framework (MOF) and could allow the dendrite-free deposition of the lithium metal. Benefiting from the uniqueness of the hetero-atom compositions and the 3D structure, the NC/Cu displayed much lower interfacial resistance and more stable Li plating/stripping behaviors compared to those of the planar Cu foil. A coulombic efficiency higher than 97% and long cycle life over 200 cycles were finely achieved with the specific capacity of 2.0 and 1.0 mA h cm−2, respectively. The merit of NC/Cu was further demonstrated in the lithium batteries versus the LiFePO4 cathode, where the cycle ability and electrochemical performances were significantly boosted. This study opens a new avenue to stabilize lithium metal anodes from the unique derivations of MOFs and more relevant researches on lithium metal chemistries deserve to be explored.


 Please wait while we load your content...
Please wait while we load your content...