Layered double hydroxide-based antioxidant dispersions with high colloidal and functional stability†
Abstract
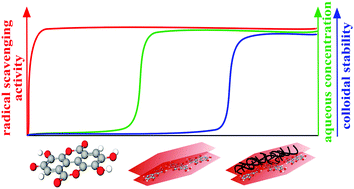
Highly stable antioxidant dispersions were designed on the basis of ring-opened ellagic acid (EA) intercalated into MgAl-layered double hydroxide (LDH) nanoparticles. The morphology of the composite was delicately modified with ethanolic washing to obtain EtOH–EA–LDH with a high specific surface area. The colloidal stability was optimized by surface functionalization with positively charged polyelectrolytes. Polyethyleneimine (PEI), protamine sulfate (PS) and poly(acrylamide-co-diallyl dimethyl ammonium chloride) (PAAm-co-DADMAC) was adsorbed onto the surface of the oppositely charged EtOH–EA–LDH leading to charge neutralization and overcharging at appropriate doses. Formation of adsorbed polyelectrolyte layers provided remarkable colloidal stability for the EtOH–EA–LDH. Modification with PEI and PAAm-co-DADMAC outstandingly improved the resistance of the particles against salt-induced aggregation with a critical coagulation concentration value above 1 M, while only limited stability was achieved by covering the nanoparticles with PS. The high antioxidant activity of EtOH–EA–LDH was greatly preserved upon polyelectrolyte coating, which was proved in the scavenging of radicals in the test reaction applied. Hence, an active antioxidant nanocomposite of high drug dose and remarkable colloidal stability was obtained to combat oxidative stress in systems of high electrolyte concentrations.


 Please wait while we load your content...
Please wait while we load your content...