Metal cation responsive anionic microgels: behaviour towards biologically relevant divalent and trivalent ions†
Abstract
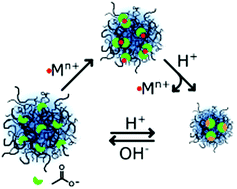
Anionic poly(vinylcaprolactam-co-itaconicacid-co-dimethylitaconate) microgels were synthesized via dispersion polymerization and their responsiveness towards cations, namely Mg2+, Sr2+, Cu2+ and Fe3+, was investigated. The itaconic moieties chelate the metal ions which act as a crosslinker and decrease the electrostatic repulsion within the network, leading to a decrease in the gel size. The responsiveness towards the metal ion concentration has been studied via dynamic light scattering (DLS) and the number of ions bonded within the network has been quantified with ion chromatography. Through the protonation of the carboxylate groups in the gel network, their interaction with the cations is significantly lowered, and the metals are consequently released back in solution. The number of ions released was assessed also via ion chromatography for all four ions, whilst Mg2+ was also used as a model ion to display the reversibility of the system. The microgels can bond and release divalent cations over multiple cycles without undergoing any loss of functionality. Moreover, these gels also selectively entrap Fe3+ with respect to the remaining divalent cations, opening the possibility of using the proposed gels in the digestive tract as biocompatible chelating agents to fight iron overaccumulation.


 Please wait while we load your content...
Please wait while we load your content...