Thorough studies of tricyanomethanide-based ionic liquids – the influence of alkyl chain length of the cation†
Abstract
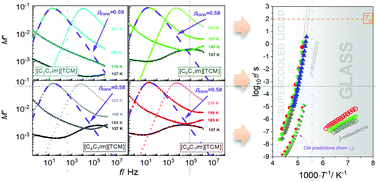
The glassy, supercooled, and normal liquid states of the 1-alkyl-3-methylimidazolium tricyanomethanide series [CnC1im][TCM] (n = 2, 4, 6, 8, and 16) were investigated by dielectric and mechanical (rheological) experiments supplemented by X-ray diffraction. The conductivity relaxation was found to be accompanied by a pronounced secondary relaxation. However, based on ambient and high-pressure results as well as the coupling model, we assumed that the latter one can not be classified as Johari–Goldstein relaxation. Moreover, the studies on the nanoscale organization of ionic liquids indicated that 1-alkyl-3-methylimidazolium tricyanomethanide ILs begin to form nanoscale aggregates when the alkyl chain of the cation has six carbon atoms.


 Please wait while we load your content...
Please wait while we load your content...