Glycosyl squaramides, a new class of supramolecular gelators†
Abstract
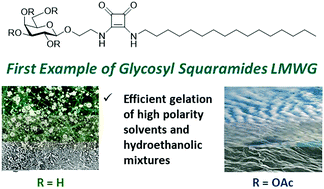
Glycosyl squaramides were synthesised and evaluated as low molecular weight gelators. Amphiphilic glycosyl squaramides 5 and 6, with a C-16 aliphatic chain, formed thermoreversible gels in polar organic solvents and 1 : 1 ethanol/water mixtures with high efficiency. Rheological analysis showed these gels achieve their structural stability 120 h after gelation and were robust, making them particularly suitable for biomedical applications. The interactions between solvent and gelator strongly influence SAFiN (Self-Assembled Fibrillar Network) formation, critical gelation concentration (CGC) and subsequent gel structure, as evidenced by SEM imaging of xerogels. Spectroscopic studies indicate that H-bonding is involved in the self-assembly of the glycosyl squaramides in organic solvents, while hydrophobic interactions are the major driving force for gel formation in the presence of water. The compounds described herein are the first reported examples of carbohydrate-squaramide conjugates capable of forming supramolecular gels.


 Please wait while we load your content...
Please wait while we load your content...