Self-assembling behaviour of a modified aromatic amino acid in competitive medium†
Abstract
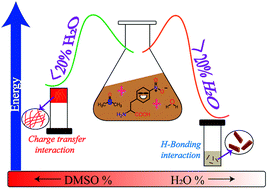
Aromatic amino acid, specifically phenylalanine (Phe), is one of the most studied building blocks in peptide synthesis due to its importance in biology. It is reported in the literature that Phe-containing peptides have a high tendency to form different self-assembled materials due to efficient aromatic–aromatic interactions. In this article, we have tuned the supramolecular interactions of phenylalanine by making it electron-deficient upon introduction of the nitro group in the ring. The presence of the nitro group has a profound influence on the self-assembly process. It has been observed that 4-nitrophenylalanine (4NP) is a highly efficient gelator compared with the native phenylalanine in DMSO solvent in terms of minimum gelation concentration and it forms hydrogen bonding mediated crystals in water. The change of self-assembling patterns of 4NP in these solvents was studied using X-ray diffraction, UV-Vis spectroscopy, FE-SEM and other techniques. With the help of different experimental data and density functional theory (DFT), we have simulated the theoretical structure of 4NP in DMSO. The theoretical structure of 4NP in DMSO is different compared with that of crystals in water. We then studied the self-assembly process of 4NP in the mixed solvent of DMSO (polar aprotic) and water (polar protic). Different competitive non-covalent interactions of solvents as well as the ratio of the solvent mixture guide the final self-assembly state of 4NP.


 Please wait while we load your content...
Please wait while we load your content...