Ion conduction in the comb-branched polyether electrolytes with controlled network structures†
Abstract
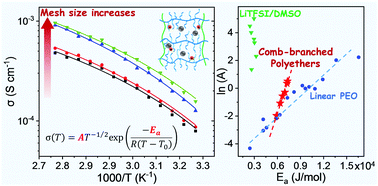
Solid polymer electrolytes (SPEs) based on centipede-like polyethers composed of short ethylene oxide (EO) brushes and allyl functional groups were generated and followed by in situ crosslinking via thiol–ene “click” chemistry. The delicate control of the mesh sizes of the networks was achieved by tuning the composition of the backbone and the length of the bi-functional EO crosslinkers, which was further evaluated by the equilibrium swelling experiments and the Flory–Rehner theory. This type of SPE demonstrates good compatibility with lithium anodes and a high ionic conductivity up to 1.6 × 10−4 S cm−1 at room temperature, 2 orders of magnitude higher than that of the typical linear PEO. The temperature dependence of the ionic conductivity can be described by the Vogel–Tammann–Fulcher (VTF) equation, which shows a systematic variation of the ion conduction behaviors with the network structures. Particularly, the increase of mesh size results in the increase of the conductivity and the decrease in the content of ion pairs, which is verified in the networks based on end-functionalized systems as well. The higher free ion content in the loose network has been attributed to its larger conformational freedom and optimized complexation of the lithium ions. This type of comb-branched polyether with solvent-like oligomer EO brushes also shows the potential to alleviate the compensation effect between the apparent activation energy and the ion carrier contents, which may provide a promising platform to fabricate high performance electrolytes with optimized ionic conductivity.


 Please wait while we load your content...
Please wait while we load your content...