Facile synthesis of Co2(OH)3Cl/cobalt carbide/reduced graphene oxide composites for enhanced dye-sensitized photocatalytic H2 evolution†‡
Abstract
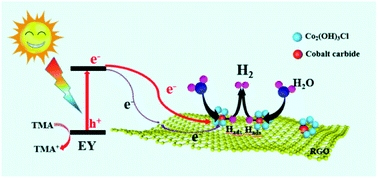
Photocatalytic H2 evolution from water splitting using catalysts has proven to be a promising strategy. In photocatalytic H2 evolution, a hydrogen evolution cocatalyst is necessary. The cocatalyst should be inexpensive and efficient. In this work, a hollow Co2(OH)3Cl/cobalt carbide/reduced graphene oxide (RGO) composite was fabricated as a cocatalyst via the one-step thermal treatment at low temperature (300 °C and 350 °C) of CoCl2/graphene oxide (GO). Cobalt carbide was formed based on the strong interaction between GO and Co2+. GO not only provides the carbon source for cobalt carbide, but also acts as a soft template of a hollow structure and promotes the distribution of active species. The obtained sample exhibits a much higher dye-sensitized photocatalytic H2 evolution activity than the catalysts prepared at high temperature (500 °C and 600 °C), which mainly consist of CoO/Co/cobalt carbide/RGO. This enhanced activity is ascribed to the formation of highly dispersed cobalt carbide and Co2(OH)3Cl, the effective electron transfer between them, and the ability of Co2(OH)3Cl to efficiently convey electrons from excited dye molecules. The highest apparent quantum yield of dye-sensitized photocatalytic H2 evolution obtained using our catalyst was 28.3% at 420 nm. The possible mechanism is also discussed.


 Please wait while we load your content...
Please wait while we load your content...