Graphdiyne formed a novel CuI-GD/g-C3N4 S-scheme heterojunction composite for efficient photocatalytic hydrogen evolution
Abstract
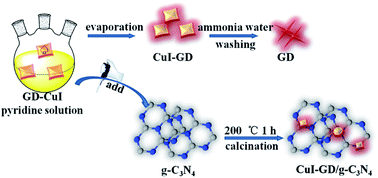
Rational design of novel and efficient hybrid photocatalysts has great significance today, as fossil energy demands urgent need to be replaced by green energy, such as the hydrogen energy. For this reason, as the youngest member of the carbon family, graphdiyne (GD) has attracted extensive attention. Herein, a new type of GD–CuI material was synthesized via a one-pot reaction. After combining GD–CuI with g-C3N4 by a simple calcination method, a step-scheme (S-scheme) heterojunction was formed between CuI–GD and g-C3N4 that substantially improved the hydrogen evolution performance of successfully synthesized CuI–GD/g-C3N4 nanocomposites. The hydrogen evolution activity of the CuI–GD/g-C3N4 photocatalyst was 9.8 times that of the pure g-C3N4 under visible-light irradiation. The introduction of CuI–GD composites prolonged the lifetime of photogenerated carriers, enhanced the electron density, and reduced the overpotential of hydrogen production, which played a positive role in enhancing the photocatalytic performance of nanocomposites. Through the in-depth analysis of the band structure of the catalyst, it could be observed that in the g-C3N4-based composite catalyst, the new materials with CuI–GD built a dual S-scheme heterojunction composite. Thus, the reasonable CuI–GD/g-C3N4 S-scheme heterojunction composite can simultaneously have the advantages of high charge separation efficiency and strong redox capacity. This work provides a new approach for the synthesis of new type of composite photocatalysts for solar energy conversion.


 Please wait while we load your content...
Please wait while we load your content...