Selective conversion of furfural into tetrahydrofurfuryl alcohol using a heteropoly acid-based material as a hydrogenation catalyst†
Abstract
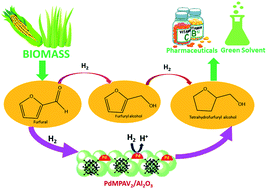
Tetrahydrofurfuryl alcohol (THFAL) is a green solvent as well as a significant platform chemical which can be obtained from biomass derived furfural. In this study, new palladium exchanged vanadium incorporated supported heteropoly molybdate (PdMPAV2) catalysts were synthesized using a simple wet impregnation method and studied for selective hydrogenation of furfural. Six different catalysts were investigated: PdMPAV2/Al2O3, PdMPAV2/TiO2, PdMPAV2/SiO2, PdMPAV2/ZrO2, PdMPAV2/Nb2O5 and Pd/Al2O3. Of the catalysts studied the PdMPAV2/Al2O3 catalyst was able to achieve the highest selectivity of THFAL (95%) under moderate reaction conditions (150 °C, 2.5 MPa, 6 h) using isopropanol as a solvent and the weight percentage of palladium is only 0.5% which is the least among all the previously reported catalysts. The other catalysts investigated achieved yields of 50–75% under the same reaction conditions. The higher selectivity obtained using the alumina supported catalyst was explained using a range of physicochemical methods: XRD, temperature programmed reduction (H2-TPR), CO pulse chemisorption, BET surface area analysis and SEM. The H2-TPR results showed that PdMPAV2/Al2O3 contained a significant amount of Pd that was reduced at the lowest temperature (∼100 °C) observed for all the materials investigated, which indicated that this catalyst most likely contained a significantly greater amount of more highly dispersed/higher surface area Pd than the other catalysts investigated which is also supported by CO pulse chemisorption results. XPS results supported the presence of Pd(0) which is stabilized by a heteropoly acid framework, and TEM and CO chemisorption results illuminated the fact that the well dispersed and smaller palladium cluster is one of the crucial factors for the better activity of PdMPAV2/Al2O3. The effects of the variation of temperature, pressure, time and catalyst concentration were investigated for the best performing catalyst (PdMPAV2/Al2O3).


 Please wait while we load your content...
Please wait while we load your content...