A thiophene bridged naphthalimide–porphyrin complex with enhanced activity and stability in photocatalytic H2 evolution†
Abstract
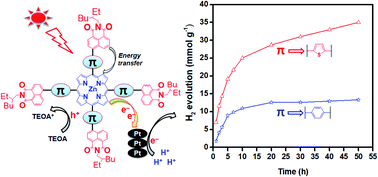
More efficient intramolecular energy transfer in the naphthalimide–porphyrin complex, ZnT(p-NI)TP, is accomplished by an electron rich coplanar thiophene π-linkage compared to the analogous porphyrin ZnT(p-NI)PP bearing a less coplanar phenylene π-linker. As a result, ZnT(p-NI)TP shows enhanced light-harvesting ability, electron lifetime and photoinduced charge carrier separation compared to ZnT(p-NI)PP and this boosted electron transfer from the photoexcited porphyrin moiety to the proton reduction catalyst, consequently, resulting in a 2.9 fold higher hydrogen evolution rate (ηH2) of ZnT(p-NI)TP (4.28 mmol g−1 h−1) than ZnT(p-NI)PP (1.50 mmol g−1 h−1). ZnT(p-NI)TP is also much more photostable than ZnT(p-NI)PP and continued to show hydrogen evolution for up to 50 h.


 Please wait while we load your content...
Please wait while we load your content...