Trace tungsten and iron-doped nickel hydroxide nanosheets for an efficient oxygen evolution reaction†
Abstract
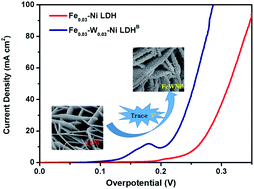
FexNi1−x(O)OH has been proven to be an efficient, earth-abundant and nonprecious catalyst for the oxygen evolution reaction (OER). However, the weak binding of oxygenated intermediates and the exposure of a few active sites on NiFe hydroxides considerably hinder their catalytic activity. Based on the fact that the W6+ ions possess higher valency. and similar ionic radius with respect to the Fe2+, Fe3+, and Ni2+ ions, which can modulate the (NiO6) crystal structure and enhance the interaction of block electrons, we have tried to introduce a trace amount of W6+ into FexNi1−x(O)OH. To our surprise, the target product Fe0.03W0.03–Ni LDHB exhibited the lowest overpotential of 205(±3) mV at 10 mA cm−2, Tafel slope of 60 mV dec−1, and no obvious decrease in the electrocatalytic activity after more than 50 hour operation or 3000 cyclic voltammetry cycles. X-ray diffraction, Raman spectroscopy, UV-Vis spectroscopy, X-ray photoelectron spectroscopy and electrochemical studies revealed a synergistic interplay among tungsten, iron and nickel for producing a favorable local coordination environment and electronic structure, which enhanced the OER performance.


 Please wait while we load your content...
Please wait while we load your content...