Electrochemically catalyzed conversion of cornstalk lignin to aromatic compounds: an integrated process of anodic oxidation of a Pb/PbO2 electrode and hydrogenation of a nickel cathode in sodium hydroxide solution
Abstract
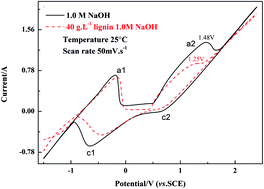
This paper presents a novel process to degrade cornstalk lignin in alkaline liquor by using an electrolytic cell, equipped with a nickel plate cathode and a Pb/PbO2 anode in sodium hydroxide solution. Cyclic voltammetry tests were conducted and polarization curves were obtained to research the electrochemical oxidation performance of the Pb/PbO2 anode and electrocatalytic hydrogenation of the Ni cathode in the lignin alkali solution. High performance liquid chromatography (HPLC) was used to characterize the functional groups and molecular weight distribution of the lignin residue. By GC-MS analysis, it was confirmed that there were 12 kinds of compounds in the degradation products of alkali cornstalk lignin. In addition to this, some commercially valuable chemicals, including toluene, m-xylene (MX), o-xylene (OX) and anisole, were also observed.


 Please wait while we load your content...
Please wait while we load your content...