Room-temperature synthesis of Ni1−xFex (oxy)hydroxides: structure–activity relationship for the oxygen evolution reaction†
Abstract
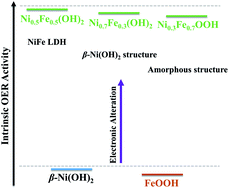
Ni–Fe (oxy)hydroxides outperform most of the non-precious metal-based catalysts towards the oxygen evolution reaction (OER). Yet, the phase structure–catalytic activity correlation remains elusive because of the difficulty in synthesizing phase-pure Ni1−xFex (oxy)hydroxides. Here, a simple room-temperature aqueous solution method has been developed to achieve phase-pure Ni1−xFex (oxy)hydroxides with Fe content ranging from 0 to 100 at%. The Ni1−xFex (oxy)hydroxides are simply derived from the metal–hydrazine complex by in situ transformation in alkaline solutions. The structural evolution and catalytic activity of the Ni1−xFex (oxy)hydroxides are comprehensively studied to reveal the relationship between the structure and catalytic activity. The Ni1−xFex (oxy)hydroxides present high activity at x = 0.3–0.7, and only a small overpotential of <260 mV is needed to achieve an anodic current density of 10 mA cm−2 in 1.0 M KOH. In addition, the catalysts exhibit high long-term working stability. It is found that their phase structure and electronic structure are related to the intrinsic activity and that the Fe/Ni ratio affects the electrical conductivity of Ni1−xFex (oxy)hydroxides for optimized performance.


 Please wait while we load your content...
Please wait while we load your content...