Catalytic conversion of ferrous carbonate to higher hydrocarbons under mild conditions and its application in transformation of CO2 to liquid fuels†
Abstract
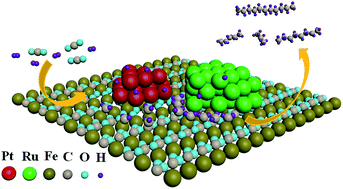
The generation of liquid fuels of higher hydrocarbons under mild conditions by consuming CO2 would bring about many economic and environmental benefits. Here we report a finding that Pt and Ru nanoparticles deposited on FeCO3 could catalyze the conversion of carbonate ions in FeCO3 and H2 to higher hydrocarbons under mild conditions. Coupling this new reaction route with the carbonation of formed surface ferrous species resulted in the conversion of CO2 and H2 to higher hydrocarbons (C2–C26) at low or even ambient temperatures due to the low activation-energy for CHx coupling in the process. At 40 °C, the selectivity for C5–C26 hydrocarbons and C2+ compounds reached 49.6% and 77.7%, respectively. This finding sheds a new light on regenerating multi-carbon energy carriers in a sustainable manner.
- This article is part of the themed collection: Sustainable Energy & Fuels Cover Art


 Please wait while we load your content...
Please wait while we load your content...