Solid-phase fluorescent BODIPY–peptide synthesis via in situ dipyrrin construction†
Abstract
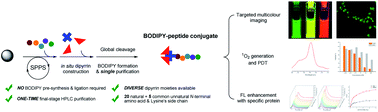
Traditional fluorescent peptide chemical syntheses hinge on the use of limited fluorescent/dye-taggable unnatural amino acids and entail multiple costly purifications. Here we describe a facile and efficient protocol for in situ construction of dipyrrins on the N-terminus with 20 natural and five unnatural amino acids and the lysine's side chain of selected peptides/peptide drugs through Fmoc-based solid-phase peptide synthesis. The new strategy enables the direct formation of boron–dipyrromethene (BODIPY)–peptide conjugates from simple aldehyde and pyrrole derivatives without pre-functionalization, and only requires a single-time chromatographic purification at the final stage. As a model study, synthesized EBNA1-targeting BODIPY1–Pep4 demonstrates intact selectivity in vitro, responsive fluorescence enhancement, and higher light cytotoxicity due to the photo-generation of cytotoxic singlet oxygen. This work offers a novel practical synthetic platform for fluorescent peptides for multifaceted biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...