Revitalizing silver nanocrystals as a redox catalyst by modifying their surface with an isocyanide-based compound†
Abstract
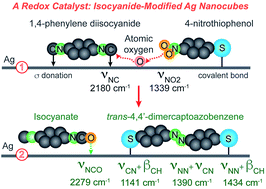
Silver is an excellent catalyst for oxidation reactions such as ethylene epoxidation, but it shows limited activity toward reduction reactions. Here we report a strategy to revitalize Ag nanocrystals as a redox catalyst for the production of an aromatic azo compound by modifying their surface with an isocyanide-based compound. We also leverage in situ fingerprint spectroscopy to acquire molecular insights into the reaction mechanism by probing the vibrational modes of all chemical species at the catalytic surface with surface-enhanced Raman spectroscopy. We establish that binding of isocyanide to Ag nanocrystals makes it possible for Ag to extract the oxygen atoms from the nitro-groups of nitroaromatics and then use these atoms to oxidize isocyanide to isocyanate. Concurrently, the coupling between two adjacent deoxygenated nitroaromatic molecules leads to the formation of an aromatic azo compound.


 Please wait while we load your content...
Please wait while we load your content...