Dynamic spatial and structural organization in artificial cells regulates signal processing by protein scaffolding†
Abstract
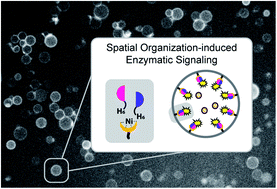
Structural and spatial organization are fundamental properties of biological systems that allow cells to regulate a wide range of biochemical processes. This organization is often transient and governed by external cues that initiate dynamic self-assembly processes. The construction of synthetic cell-like materials with similar properties requires the hierarchical and reversible organization of selected functional components on molecular scaffolds to dynamically regulate signaling pathways. The realization of such transient molecular programs in synthetic cells, however, remains underexplored due to the associated complexity of such hierarchical platforms. In this contribution, we effectuate dynamic spatial organization of effector protein subunits in a synthetic biomimetic compartment, a giant unilamellar vesicle (GUV), by associating in a reversible manner two fragments of a split luciferase to the membrane. This induces their structural dimerization, which consequently leads to the activation of enzymatic signaling. Importantly, such organization and activation are dynamic processes, and can be autonomously regulated – thus opening up avenues toward continuous spatiotemporal control over supramolecular organization and signaling in an artificial cell.
- This article is part of the themed collections: Editor’s Choice – Subi George and Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...