Biosynthesis of the fungal glyceraldehyde-3-phosphate dehydrogenase inhibitor heptelidic acid and mechanism of self-resistance†
Abstract
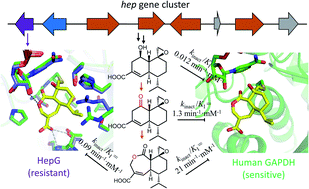
Overcoming resistance to bioactive small molecules is a significant challenge for health care and agriculture. As a result, efforts to uncover the mechanisms of resistance are essential to the development of new antibiotics, anticancer drugs and pesticides. To study how nature evolves resistance to highly potent natural products, we examined the biosynthesis and mechanism of self-resistance of the fungal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) inhibitor heptelidic acid (HA). HA is a nanomolar inhibitor of GADPH through the covalent modification of the active site cysteine thiol. The biosynthetic pathway of HA was elucidated, which uncovered the enzymatic basis of formation of the epoxide warhead. Structure–activity relationship study using biosynthetic intermediates established the importance of the fused lactone ring system in HA. The molecular basis of HA inhibiting human GAPDH was illustrated through the crystal structure of Hs-GAPDH covalently bound with HA. A GAPDH isozyme HepG encoded in the HA cluster was characterized to be less sensitive to HA, and therefore contribute to self-resistance for the producing host. Comparison of the crystal structures of human GAPDH and HepG showed mutations both within and remote to the active site can contribute to resistance of inactivation, which was confirmed through mutagenesis. Due to the critical role GAPDH plays in aerobic glycolysis and other cellular functions, knowledge of HA mode of action and self-resistance mechanism could accelerate the development of improved inhibitors.


 Please wait while we load your content...
Please wait while we load your content...