Asymmetric synthesis of γ-chiral borylalkanes via sequential reduction/hydroboration using a single copper catalyst†
Abstract
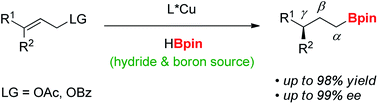
The synthesis of γ-chiral borylalkanes through copper-catalyzed enantioselective SN2′-reduction of γ,γ-disubstituted allylic substrates and subsequent hydroboration was reported. A copper–DTBM-Segphos catalyst produced a range of γ-chiral alkylboronates from easily accessible allylic acetate or benzoate with high enantioselectivities up to 99% ee. Furthermore, selective organic transformations of the resulting γ-chiral alkylboronates generated the corresponding γ-chiral alcohol, arene and amine compounds.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...