Using light intensity to control reaction kinetics and reversibility in photomechanical crystals†
Abstract
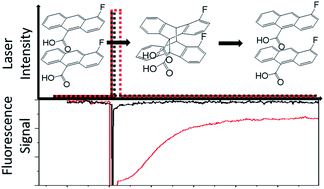
4-Fluoro-9-anthracenecarboxylic acid (4F-9AC) is a thermally reversible (T-type) photomechanical molecular crystal. The photomechanical response is driven by a [4 + 4] photodimerization reaction, while the photodimer dissociation determines the reset time. In this paper, both the chemical kinetics of dimer dissociation (using a microscopic fluorescence-recovery-after-photobleaching experiment) and mechanical reset dynamics (by imaging bending microneedles) for single 4F-9AC crystals are measured. The dissociation kinetics depend strongly on the initial concentration of photodimer, slowing down and becoming nonexponential at high dimer concentrations. This dose-dependent behavior is also observed in the mechanical response of bending microneedles. A new feature in the photomechanical behavior is identified: the ability of a very weak control beam to suppress dimer dissociation after large initial dimer conversions. This phenomenon provides a way to optically control the mechanical response of this photomechanical crystal. To gain physical insight into the origin of the nonexponential recovery curves, the experimental results are analyzed in terms of a standard first-order kinetic model and a nonlinear Finke–Watzky (FW) model. The FW model can qualitatively reproduce the transition from exponential to sigmoidal recovery with larger initial conversions, but neither model can reproduce the suppression of the recovery in the presence of a weak holding beam. These results highlight the need for more sophisticated theories to describe cooperative phenomena in solid-state crystalline reactions, as well as demonstrating how this behavior could lead to new properties and/or improved performance in photomechanical materials.


 Please wait while we load your content...
Please wait while we load your content...