Mechanism and origins of selectivity in the enantioselective oxa-Pictet–Spengler reaction: a cooperative catalytic complex from a hydrogen bond donor and chiral phosphoric acid†
Abstract
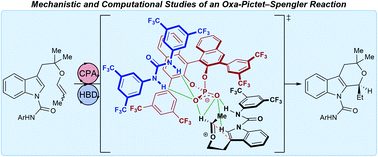
Enantioselective additions to oxocarbenium ions are high-value synthetic transformations but have proven challenging to achieve. In particular, the oxa-Pictet–Spengler reaction has only recently been rendered enantioselective. We report experimental and computational studies on the mechanism of this unusual transformation. Herein we reveal that this reaction is hypothesized to proceed through a self-assembled ternary hydrogen bonding complex involving the substrate, chiral phosphate ion, and a urea hydrogen-bond donor. The computed transition state reveals C2-symmetric grooves in the chiral phosphate that are occupied by the urea and substrate. Occupation of one of these grooves by the urea co-catalyst tunes the available reactive volume and enhances the stereoselectivity of the chiral phosphate catalyst.


 Please wait while we load your content...
Please wait while we load your content...