Enthalpic and entropic contributions to the basicity of cycloalkylamines†
Abstract
Large-ring cycloalkylamines are slightly less basic than other cycloalkylamines such as cyclohexylamine, even though all have tetrahedral carbons and are strain-free. To understand why, enthalpy and entropy for protonation of a series of cycloalkylamines were accurately determined by isothermal titration calorimetry in 3 : 1 methanol–water. The study required resolving a discrepancy between these measurements and those in pure water. The data show that the lower basicity of large-ring cycloalkylamines is not due to enthalpy but to a more negative entropy of protonation. Computations show that this can be attributed in part to an entropy of conformational mixing, but the dominant contribution is steric hindrance to solvation, also corroborated by computation.
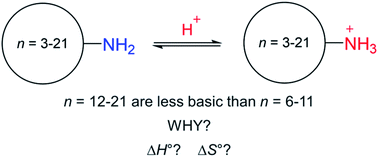


 Please wait while we load your content...
Please wait while we load your content...