The key role of R–NHC coupling (R = C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species
Abstract
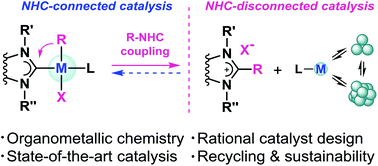
Complexes of metals with N-heterocyclic carbene ligands (M/NHC) are typically considered the systems of choice in homogeneous catalysis due to their stable metal–ligand framework. However, it becomes obvious that even metal species with a strong M–NHC bond can undergo evolution in catalytic systems, and processes of M–NHC bond cleavage are common for different metals and NHC ligands. This review is focused on the main types of the M–NHC bond cleavage reactions and their impact on activity and stability of M/NHC catalytic systems. For the first time, we consider these processes in terms of NHC-connected and NHC-disconnected active species derived from M/NHC precatalysts and classify them as fundamentally different types of catalysts. Problems of rational catalyst design and sustainability issues are discussed in the context of the two different types of M/NHC catalysis mechanisms.


 Please wait while we load your content...
Please wait while we load your content...