Enantio- and diastereoselective conjugate borylation/Mannich cyclization†
Abstract
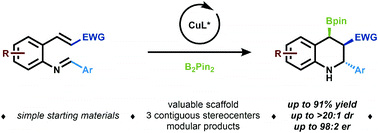
Strategies to capitalize on enolate intermediates generated from stereoselective conjugate borylation to α,β-unsaturated carbonyl systems are surprisingly rare despite the ubiquity of Michael acceptors, and the potential to generate valuable scaffolds bearing multiple stereocenters. Herein, we report a mild and stereoselective copper-catalyzed conjugate borylation/Mannich cyclization reaction. This strategy is feasible with a broad range of Michael acceptors, and can be leveraged to generate versatile borylated tetrahydroquinoline scaffolds bearing three contiguous stereocenters. The synthetic potential of these complex heterocycles has been explored through a series of derivatization studies.


 Please wait while we load your content...
Please wait while we load your content...