Anodic oxidation triggered divergent 1,2- and 1,4-group transfer reactions of β-hydroxycarboxylic acids enabled by electrochemical regulation†
Abstract
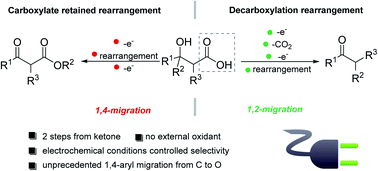
We report a set of electrochemically regulated protocols for the divergent synthesis of ketones and β-keto esters from the same β-hydroxycarboxylic acid starting materials. Enabled by electrochemical control, the anodic oxidation of carboxylic acids proceeded in either a one-electron or a two-electron pathway, leading to a 1,4-aryl transfer or a semipinacol-type 1,2-group transfer product with excellent chemoselectivity. The 1,4-aryl transfer represents an unprecedented example of carbon-to-oxygen group transfer proceeding via a radical mechanism. In contrast to previously reported radical group transfer reactions, this 1,4-group transfer process features the migration of electron-rich aryl substituents. Furthermore, with these chemoselective electrochemical oxidation protocols, a range of ketones and β-keto esters including those possessing a challenging-to-access medium-sized ring could be synthesized in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...