A unified strategy to reverse-prenylated indole alkaloids: total syntheses of preparaherquamide, premalbrancheamide, and (+)-VM-55599†‡
Abstract
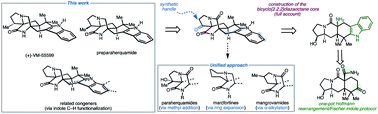
A full account of our studies toward reverse-prenylated indole alkaloids that contain a bicyclo[2.2.2]core is described. A divergent route is reported which has resulted in the synthesis of preparaherquamide, (+)-VM-55599, and premalbrancheamide. An intramolecular Dieckmann cyclization between an enolate and isocyanate was used to forge the bicyclo[2.2.2]diazaoctane core that is characteristic of these molecules. The pentacyclic indole scaffold was constructed through a one-pot Hofmann rearrangement followed by Fischer indole synthesis. The utilization of our previously reported indole peripheral functionalization strategy also led to natural products including malbrancheamides B, C, stephacidin A, notoamides F, I and R, aspergamide B, and waikialoid A. Ultimately, the divergent route that we devised provided access to a wide range of prenylated indole alkaloids that are differently substituted on the cyclic amine core.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...