Fe-catalyzed three-component dicarbofunctionalization of unactivated alkenes with alkyl halides and Grignard reagents†
Abstract
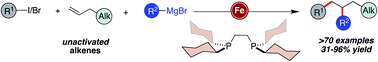
A highly chemoselective iron-catalyzed three-component dicarbofunctionalization of unactivated olefins with alkyl halides (iodides and bromides) and sp2-hybridized Grignard reagents is reported. The reaction operates under fast turnover frequency and tolerates a diverse range of sp2-hybridized nucleophiles (electron-rich and electron-deficient (hetero)aryl and alkenyl Grignard reagents), alkyl halides (tertiary alkyl iodides/bromides and perfluorinated bromides), and unactivated olefins bearing diverse functional groups including tethered alkenes, ethers, protected alcohols, aldehydes, and amines to yield the desired 1,2-alkylarylated products with high regiocontrol. Further, we demonstrate that this protocol is amenable for the synthesis of new (hetero)carbocycles including tetrahydrofurans and pyrrolidines via a three-component radical cascade cyclization/arylation that forges three new C–C bonds.


 Please wait while we load your content...
Please wait while we load your content...