Synthesis of oxalamides by acceptorless dehydrogenative coupling of ethylene glycol and amines and the reverse hydrogenation catalyzed by ruthenium†
Abstract
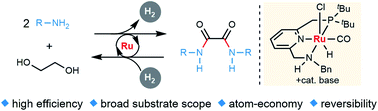
A sustainable, new synthesis of oxalamides, by acceptorless dehydrogenative coupling of ethylene glycol with amines, generating H2, homogeneously catalyzed by a ruthenium pincer complex, is presented. The reverse hydrogenation reaction is also accomplished using the same catalyst. A plausible reaction mechanism is proposed based on stoichiometric reactions, NMR studies, X-ray crystallography as well as observation of plausible intermediates.


 Please wait while we load your content...
Please wait while we load your content...