Build-up of double carbohelicenes using nitroarenes: dual role of the nitro functionality as an activating and leaving group†
Abstract
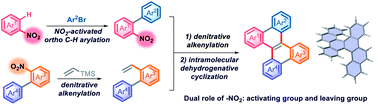
The construction of double carbohelicenes is highly fascinating yet challenging work. Disclosed herein is a streamlined and simplified synthetic route to double carbohelicenes starting from nitroarenes through sequential nitro-activated ortho-C–H arylation, denitrative alkenylation and intramolecular cyclodehydrogenation. In this synthetic strategy, the nitro group plays a dual role namely as a leaving group for the denitrative alkenylation and as an activating group for ortho-C–H arylation, which is distinct from those of aryl halides in a conventional coupling reaction. In this work, the palladium-catalyzed Heck-type alkenylation of nitroarenes has been presented, in which the conventionally inert Ar–NO2 bond is cleaved. This work provides a novel synthetic strategy for polycyclic aromatic hydrocarbons (PAHs).


 Please wait while we load your content...
Please wait while we load your content...