Ti-catalyzed ring-opening oxidative amination of methylenecyclopropanes with diazenes†
Abstract
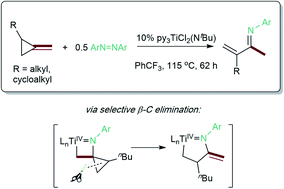
The ring-opening oxidative amination of methylenecyclopropanes (MCPs) with diazenes catalyzed by py3TiCl2(NR) complexes is reported. This reaction selectively generates branched α-methylene imines as opposed to linear α,β-unsaturated imines, which are difficult to access via other methods. Products can be isolated as the imine or hydrolyzed to the corresponding ketone in good yields. Mechanistic investigation via density functional theory suggests that the regioselectivity of these products results from a Curtin–Hammett kinetic scenario, where reversible β-carbon elimination of a spirocyclic [2 + 2] azatitanacyclobutene intermediate is followed by selectivity-determining β-hydrogen elimination of the resulting metallacycle. Further functionalizations of these branched α-methylene imine products are explored, demonstrating their utility as building blocks.


 Please wait while we load your content...
Please wait while we load your content...