A visible-light Paternò–Büchi dearomatisation process towards the construction of oxeto-indolinic polycycles†
Abstract
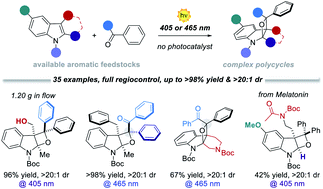
A variety of highly functionalised N-containing polycycles (35 examples) are synthesised from simple indoles and aromatic ketones through a mild visible-light Paternò–Büchi process. Tetrahydrooxeto[2,3-b]indole scaffolds, with up to three contiguous all-substituted stereocenters, are generated in high yield (up to >98%) and excellent site- regio- and diastereocontrol (>20 : 1). The use of visible light (405 or 465 nm) ensures enhanced performances by switching off undesired photodimerisation side reactions. The reaction can be easily implemented using a microfluidic photoreactor with improved productivity (up to 0.176 mmol h−1) and generality. Mechanistic investigations revealed that two alternative reaction mechanisms can account for the excellent regio- and diastereocontrol observed.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...