Palladium-catalyzed synthesis of β-hydroxy compounds via a strained 6,4-palladacycle from directed C–H activation of anilines and C–O insertion of epoxides†
Abstract
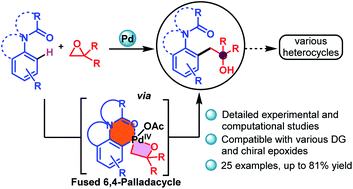
A palladium-catalyzed C–H activation of acetylated anilines (acetanilides, 1,1-dimethyl-3-phenylurea, 1-phenylpyrrolidin-2-one, and 1-(indolin-1-yl)ethan-1-one) with epoxides using O-coordinating directing groups was accomplished. This C–H alkylation reaction proceeds via formation of a previously unknown 6,4-palladacycle intermediate and provides rapid access to regioselectively functionalized β-hydroxy products. Notably, this catalytic system is applicable for the gram scale mono-functionalization of acetanilide in good yields. The palladium-catalyzed coupling reaction of the ortho-C(sp2) atom of O-coordinating directing groups with a C(sp3) carbon of chiral epoxides offers diverse substrate scope in good to excellent yields. In addition, further transformations of the synthesized compound led to biologically important heterocycles. Density functional theory reveals that the 6,4-palladacycle leveraged in this work is significantly more strained (>10 kcal mol−1) than the literature known 5,4 palladacycles.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...