Exploiting single-electron transfer in Lewis pairs for catalytic bond-forming reactions†
Abstract
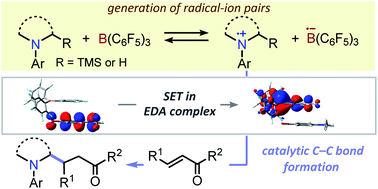
A single-electron transfer (SET) between tris(pentafluorophenyl)borane (B(C6F5)3) and N,N-dialkylanilines is reported, which is operative via the formation of an electron donor–acceptor (EDA) complex involving π-orbital interactions as a key intermediate under dark conditions or visible-light irradiation depending on the structure of the aniline derivatives. This inherent SET in the Lewis pairs initiates the generation of the corresponding α-aminoalkyl radicals and their additions to electron-deficient olefins, revealing the ability of B(C6F5)3 to act as an effective one-electron redox catalyst.


 Please wait while we load your content...
Please wait while we load your content...