Self-enhanced multicolor electrochemiluminescence by competitive electron-transfer processes†
Abstract
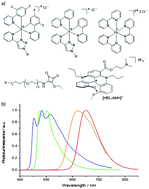
Controlling electrochemiluminescence (ECL) color(s) is crucial for many applications ranging from multiplexed bioassays to ECL microscopy. This can only be achieved through the fundamental understanding of high-energy electron-transfer processes in complex and competitive reaction schemes. Recently, this field has generated huge interest, but the effective implementation of multicolor ECL is constrained by the limited number of ECL-active organometallic dyes. Herein, the first self-enhanced organic ECL dye, a chiral red-emitting cationic diaza [4]helicene connected to a dimethylamino moiety by a short linker, is reported. This molecular system integrates bifunctional ECL features (i.e. luminophore and coreactant) and each function may be operated either separately or simultaneously. This unique level of control is enabled by integrating but decoupling both molecular functions in a single molecule. Through this dual molecular reactivity, concomitant multicolor ECL emission from red to blue with tunable intensity is readily obtained in aqueous media. This is done through competitive electron-transfer processes between the helicene and a ruthenium or iridium dye. The reported approach provides a general methodology to extend to other coreactant/luminophore systems, opening enticing perspectives for spectrally distinct detection of several analytes, and original analytical and imaging strategies.


 Please wait while we load your content...
Please wait while we load your content...