Strategies for remote enantiocontrol in chiral gold(iii) complexes applied to catalytic enantioselective γ,δ-Diels–Alder reactions†
Abstract
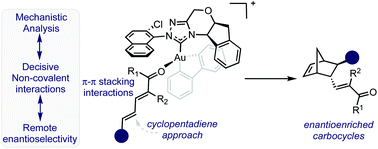
The use of chiral square planar gold(III) complexes to access enantioenriched products has rarely been applied in asymmetric catalysis. In this context, we report a mechanistic and synthetic investigation into the use of N-heterocyclic (NHC) gold(III) complexes in γ,δ-Diels–Alder reactions of 2,4-dienals with cyclopentadiene. The optimal catalyst bearing a unique 2-chloro-1-naphthyl substituent allowed efficient synthesis of functionally rich carbocycles in good yields, diastereo- and enantioselectivities. Transition state and multivariate linear regression (MLR) analysis of both catalyst and substrate trends using molecular descriptors derived from designer parameter acquisition platforms, reveals attractive non-covalent interactions (NCIs) to be key selectivity determinates. These analyses demonstrate that a putative π–π interaction between the substrate proximal double bond and the catalyst aromatic group is an essential feature for high enantioselectivity.
- This article is part of the themed collections: Celebrating 10 years of Chemical Science and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...