Enantio- and diastereoselective diarylmethylation of 1,3-dicarbonyl compounds†
Abstract
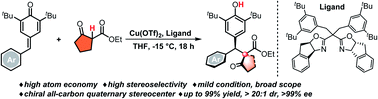
A Cu-catalyzed enantio- and diastereoselective diarylmethylation of 1,3-dicarbonyl compounds is described. It is a successful example of constructing all-carbon quaternary stereocenters from 3 °C–H nucleophiles, a challenging topic in synthetic chemistry. In the present work, two contiguous stereocenters are constructed with high levels of stereoselectivity and atom economy. The broad scope of 1,3-dicarbonyl nucleophiles and the tolerance of a wide range of functional groups make this protocol of great importance in the synthesis of chiral diarylmethane compounds.


 Please wait while we load your content...
Please wait while we load your content...