Visible-light photooxidation in water by 1O2-generating supramolecular hydrogels†
Abstract
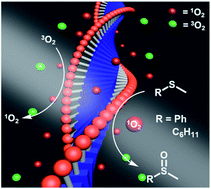
We report supramolecular photocatalytic hydrogels, produced by the enzymatically driven self-assembly of low molecular weight gelators (LMWGs). These LMWG precursors are composed of the organic chromophore diketopyrrolopyrrole (DPP), which is bi-functionalized with a series of amino acid (Phe, Tyr, Leu) methyl esters. In situ enzymatic hydrolysis of these photoactive precursors results in supramolecular hydrogels that provide a high density of photocatalytic sites. Under visible light irradiation these hydrophobic fibers recruit the reaction substrates and also produce 1O2, which is used here for the photooxidation of thioanisole (aromatic substrate) and cyclohexyl methyl sulfide (aliphatic substrate), with yields as high as 100% and without over-oxidation. Finally, we demonstrate that the nature of the amino acids in the LWMGs has a central role in dictating J-/H-/mixed state aggregates, gel properties, and, hence, the efficiency of chemoselective photooxidation of thioanisole and cyclohexyl methyl sulfide inside these hydrogels.


 Please wait while we load your content...
Please wait while we load your content...