Reversible reduction drives anion ejection and C60 binding within an Fe II4L6 cage†
Abstract
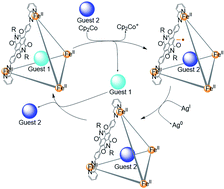
FeII4L6 tetrahedral cage 1 was prepared from a redox-active dicationic naphthalenediimide (NDI) ligand. The +20 charge of the cage makes it a good host for anionic guests, with no binding observed for neutral aromatic molecules. Following reduction by Cp2Co, the cage released anionic guests; subsequent oxidation by AgNTf2 led to re-uptake of anions. In its reduced form, however, 1 was observed to bind neutral C60. The fullerene guest was subsequently ejected following cage re-oxidation. The guest release process was found to be facilitated by anion-mediated transport from organic to aqueous solution. Cage 1 thus employs electron transfer as a stimulus to control the uptake and release of both neutral and charged guests, through distinct pathways.


 Please wait while we load your content...
Please wait while we load your content...