Kinetically controlled Ag+-coordinated chiral supramolecular polymerization accompanying a helical inversion†
Abstract
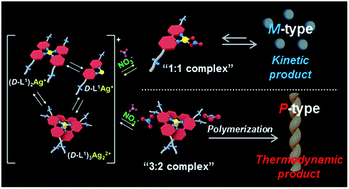
We report kinetically controlled chiral supramolecular polymerization based on ligand–metal complex with a 3 : 2 (L : Ag+) stoichiometry accompanying a helical inversion in water. A new family of bipyridine-based ligands (D-L1, L-L1, D-L2, and D-L3) possessing hydrazine and D- or L-alanine moieties at the alkyl chain groups has been designed and synthesized. Interestingly, upon addition of AgNO3 (0.5–1.3 equiv.) to the D-L1 solution, it generated the aggregate I composed of the D-L1AgNO3 complex (D-L1 : Ag+ = 1 : 1) as the kinetic product with a spherical structure. Then, aggregate I (nanoparticle) was transformed into the aggregate II (supramolecular polymer) based on the (D-L1)3Ag2(NO3)2 complex as the thermodynamic product with a fiber structure, which led to the helical inversion from the left-handed (M-type) to the right-handed (P-type) helicity accompanying CD amplification. In contrast, the spherical aggregate I (nanoparticle) composed of the D-L1AgNO3 complex with the left-handed (M-type) helicity formed in the presence of 2.0 equiv. of AgNO3 and was not additionally changed, which indicated that it was the thermodynamic product. The chiral supramolecular polymer based on (D-L1)3Ag2(NO3)2 was produced via a nucleation–elongation mechanism with a cooperative pathway. In thermodynamic study, the standard ΔG° and ΔHe values for the aggregates I and II were calculated using the van't Hoff plot. The enhanced ΔG° value of the aggregate II compared to that of the formation of aggregate I confirms that aggregate II was thermodynamically more stable. In the kinetic study, the influence of concentration of AgNO3 confirmed the initial formation of the aggregate I (nanoparticle), which then evolved to the aggregate II (supramolecular polymer). Thus, the concentration of the (D-L1)3Ag2(NO3)2 complex in the initial state plays a critical role in generating aggregate II (supramolecular polymer). In particular, NO3− acts as a critical linker and accelerator in the transformation from the aggregate I to the aggregate II. This is the first example of a system for a kinetically controlled chiral supramolecular polymer that is formed via multiple steps with coordination structural change.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...