A continuous-flow approach for the multi-gram scale synthesis of C2-alkyl- or β-amino functionalized 1,3-dicarbonyl derivatives and ondansetron drug using 1,3-dicarbonyls†
Abstract
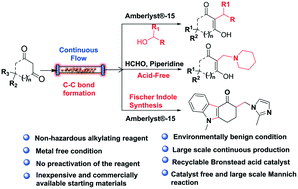
Continuous-flow chemistry is a modern technology that encompasses the green chemistry principles for the multi-gram synthesis of various API and drugs. Herein, we have developed a highly efficient and environmentally benign metal-free alkylation of 1,3-dicarbonyl compounds using secondary alcohols in the presence of inexpensive Amberlyst®-15 under continuous-flow. This method has a broad substrate scope with a variety of secondary alcohols and water as a byproduct. The Amberlyst®-15 is recyclable and reusable for the alkylation reaction under batch/continuous-flow technology. Furthermore, a continuous-flow technology driven Mannich reaction was demonstrated under an acid-free condition. In addition, a continuous-flow Fischer indole strategy for the ondansetron with an improved yield was demonstrated. Additionally, all these reactions were demonstrated with multi-gram scale synthesis without lowering the yield under batch/continuous-flow technology.


 Please wait while we load your content...
Please wait while we load your content...