Arsenic immobilization as crystalline scorodite by gas-diffusion electrocrystallization†
Abstract
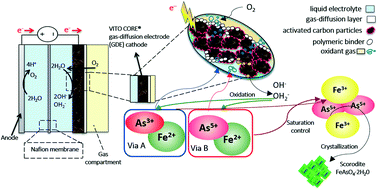
The safe immobilization of arsenic present in liquids is a key environmental challenge due to the inherent toxicity of arsenic. This immobilization is mostly restricted by the application of chemicals and several stages of oxidation and precipitation. Although the formation of bioscorodite is a greener alternative, it is intensive in the use of energy for aeration, and it is costly due to nutrient addition. The electrochemically-driven crystallization of arsenic into scorodite is proposed here to overcome these limitations. We disclose gas-diffusion electrocrystallization (GDEx) for the immobilization of arsenic into highly crystalline scorodite (FeAsO4·2H2O) by the in situ production of oxidizing substances (i.e., H2O2) on gas-diffusion electrodes. GDEx yielded an exceptional arsenic immobilization efficiency of up to 70% without the use of any primary minerals or seed crystals. At 70 °C and using As3+ as the precursor, polydisperse micrometric scorodite particles were obtained (from fine particles of <1 μm to large particles of ∼5 μm). In contrast, fine micrometric particles of <1 μm were achieved using As5+ as the precursor. Using one-pot and one-step GDEx enabled the synthesis of scorodite that was 14 times less soluble than required for stable scorodite disposal. Current chemical oxidation–precipitation processes use two separate reactors, including the oxidation of As3+ to As5+, and then the precipitation of the As5+ with Fe3+ to generate scorodite at a temperature higher than 90 °C. In contrast, the new GDEx approach combines both reactors into one to produce crystalline scorodite at 50 °C, hence reducing energy requirements and chemical footprint.


 Please wait while we load your content...
Please wait while we load your content...