Influence of the acid–base stoichiometry and residual water on the transport mechanism in a highly-Brønsted-acidic proton-conducting ionic liquid
Abstract
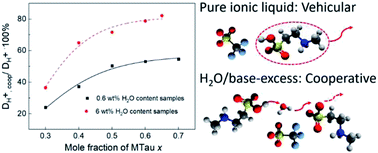
In this study, Brønsted-acidic proton conducting ionic liquids are considered as potential new electrolytes for polymer membrane fuel cells with operating temperatures above 100 °C. N-Methyltaurine and trifluoromethanesulfonic acid (TfOH) were mixed at various stoichiometric ratios in order to investigate the influence of an acid or base excess. The proton conductivity and self-diffusion of the “neat” and with 6 wt% water samples were investigated by following electrochemical and NMR methods. The composition change in the complete species and the relative proton transport mechanism based on the NMR results are discussed in detail. During fuel cell operation, the presence of significant amounts of residual water is unavoidable. In PEFC electrolytes, the predominating proton transfer process depends on the cooperative mechanism, when PILs are fixed on the polymer matrix within the membrane. Due to the comparable acidity of the cation [2-Sema]+ and the hydroxonium cation, with excess N-methyltaurine or H2O in the compositions, fast proton exchange reactions between the protonated [2-Sema]+ cation, N-methyltaurine and H2O can be envisaged. Thus, an increasing ratio of cooperative proton transport could be observed. Therefore, for polymer membrane fuel cells operating at elevated temperatures, the highly acidic PILs with excess bases are promising candidates for future use as electrolytes.


 Please wait while we load your content...
Please wait while we load your content...