The potential role of the 5,6-dihydropyridin-2(1H)-one unit of piperlongumine on the anticancer activity†
Abstract
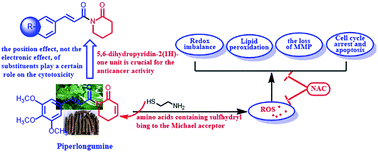
Piperlongumine (PL), a potent anticancer agent from the plant long pepper (Piper longum), contains the 5,6-dihydropyridin-2(1H)-one heterocyclic scaffold and cinnamoyl unit. In this paper, we synthesized a series of PL analogs and evaluated their cytotoxicity against cancer cells for the sake of exploring which pharmacophore plays a more potent role in enhancing the anticancer activities of PL. These results illustrated that the position effect, not the electronic effect, of substituents plays a certain role in the cytotoxicity of PL and its analogs. More important, the 5,6-dihydropyridin-2(1H)-one unit, a potent pharmacophore in enhancing the antiproliferative activities of PL, could react with cysteamine and lead to ROS generation, and then bring about the occurrence of ROS-induced downstream events, followed by cell cycle arrest and apoptosis. This work suggests that introducing a lactam unit containing Michael acceptors may be a potent strategy to enhancing the anticancer activity of drugs.


 Please wait while we load your content...
Please wait while we load your content...