Selective leaching of lead from lead smelter residues using EDTA†
Abstract
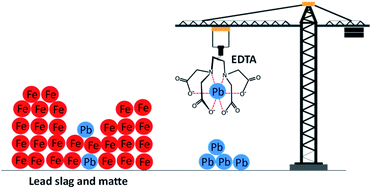
Ethylenediaminetetraacetic acid (EDTA) has been widely used as an effective reagent for removal of lead from soil because of its high lead extraction efficiency caused by the high thermodynamic stability of the Pb(II)–EDTA complex. In this study, EDTA was used as a lixiviant for recovery of lead from residues (matte and slag) of secondary lead smelter plants. The residues were composed mainly of iron (34–66 wt%) and lead (7–11 wt%). Leaching parameters (EDTA concentration, pH, temperature, liquid-to-solid ratio and leaching time) were optimized. The optimum leaching efficiency was achieved when leached for 1 h at room temperature using 0.05 mol L−1 EDTA at a liquid-to-solid ratio of 5 mL g−1. At such conditions, 72 to 80% of lead and less than 1% of iron were leached from both matte and slag. The high selectivity towards lead with minimal co-dissolution of iron is a major advantage since it reduces the chemical consumption and simplifies the downstream processes. Although the stability constants of the complexes Fe(III)–EDTA, Fe(II)–EDTA and Pb–EDTA are all large (log KS 25.1, 14.33 and 18.04, respectively), the leaching of iron was most likely limited by its presence in insoluble phases such as iron oxides, sulfides and silicates in the residues. 100% leaching of lead was achieved by a multi-step leaching process where the leaching residues were contacted three times by a fresh EDTA solution. To recover EDTA, first iron was precipitated as iron hydroxide by raising the pH of pregnant leach solution (PLS) above 12.6 using sodium hydroxide, followed by precipitation of lead as lead sulfide by adding ammonium sulfide. The recovered EDTA was successfully reused two times for leaching without significant changes in leaching yields.


 Please wait while we load your content...
Please wait while we load your content...