Material balance in the O2 electrode of Li–O2 cells with a porous carbon electrode and TEGDME-based electrolytes†
Abstract
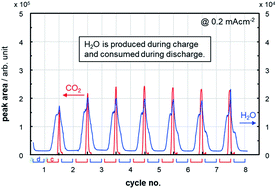
This work figures out the material balance of the reactions occurring in the O2 electrode of a Li–O2 cell, where a Ketjenblack-based porous carbon electrode comes into contact with a tetraethylene glycol dimethyl ether (TEGDME)-based electrolyte under more practical conditions of less electrolyte amount and high areal capacity. The ratio of electrolyte weight to cell capacity (E/C, g A h−1) is a good parameter to correlate with cycle life. Only 5 cycles were obtained at an areal capacity of 4 mA h cm−2 (E/C = 10) and a discharge/charge current density of 0.4 mA cm−2, which corresponds to the energy density of 170 W h kg−1 at a complete cell level. When the areal capacity was decreased to half (E/C = 20) by setting a current density at 0.2 mA cm−2, the cycle life was extended to 18 cycles. However, the total electric charge consumed for parasitic reactions was 35 and 59% at the first and the third cycle, respectively. This surprisingly large amount of parasitic reactions was suppressed by half using redox mediators at 0.4 mA cm−2 while keeping a similar cycle life. Based on by-product distribution, we will propose possible mechanisms of TEGDME decomposition and report a water breathing behavior, where H2O is produced during charge and consumed during discharge.


 Please wait while we load your content...
Please wait while we load your content...