Possible dissolution mechanism of alkali lignin in lactic acid-choline chloride under mild conditions†
Abstract
In this study, several representative deep eutectic solvents (DESs) were designed to evaluate the solubility for alkali lignin (AL). It was found that DESs with lactic acid (LA) as hydrogen bond donors (HBDs) had good solubility for AL, in which lactic acid(LA) : choline chloride(ChCl) 10 : 1 showed excellent solubility with more than 17 wt% under a relatively mild condition of 60 °C. The results of gel permeation chromatography (GPC), FTIR and 1H and HSQC NMR spectroscopies revealed an important possible dissolution mechanism of AL in LA-ChCl, that is, AL could be depolymerized under the action of LA when dissolved in LA-ChCl. Then, a new connection would form between the phenolic groups on the lignin fragments and ChCl, which is similar to that between ChCl and LA in DES, leading to an increase in the molecule weight of lignin. The new connections could be easily broken under the action of heat (150 °C) or microwave to the redispersion of lignin fragments. The results would provide a theoretic base for the high-value application of lignin in bioresources.
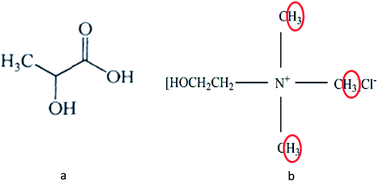


 Please wait while we load your content...
Please wait while we load your content...